
Overview:
Products Details:
Applications:
Resources:
Operating Principle
A simple Heat Pipe is a hollow element partially filled with a working fluid and has two sections, an evaporator and a condenser. Evaporator absorbs heat and uses it to convert working fluid to vapor, which expands to fill the entire internal space of the heat pipe. The condenser is in contact with heat sink and rejects heat leading to the vapor losing the heat of condensation and converting back to liquid. The liquid returns to evaporator and absorbs heat again to evaporate and the cycle repeats.
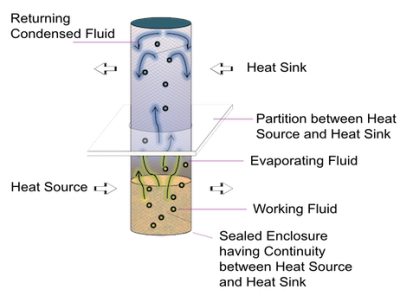
The Magic of Phase Change
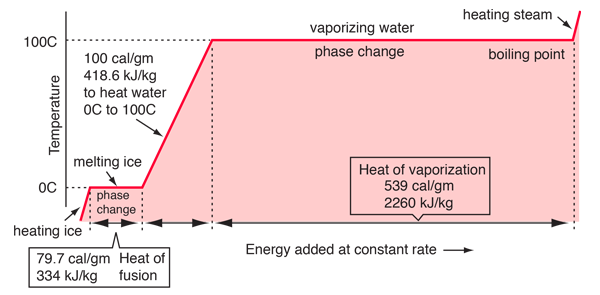
The heat pipes are unique because of they use the latent heat of working fluid in performing the job of a mule soaking up the heat from heat source and then release it to heat sink.
With phase change in action during heat pick up, the delta T required for action to begin is tiny. Moreover while the heat pick up happens, the working fluid remains at constant temperature. As compared to water for example, when liquid water picks up heat its temperature keeps going up, reducing the delta T gap that powers the heat transfers. In phase change heat transfer, since the temperature of fluid does not change, the heat transfer capacities per unit fluid weight are enhanced greatly.
